Melnyk Levels of Evidence
The Melnyk levels of evidence represent a crucial framework in evidence-based practice (EBP) that helps healthcare professionals evaluate and rank research evidence. Developed by Bernadette Melnyk and Ellen Fineout-Overholt, these levels of evidence assist nurses and researchers in making informed clinical decisions. As a systematic approach to categorizing research studies, the Melnyk levels of evidence provide a reliable method for assessing the strength and quality of available research in nursing and healthcare.
This comprehensive guide aims to help nursing students and healthcare professionals understand and adopt the Melnyk levels of evidence in their practice. Whether you’re conducting research at a university library, working in a clinical setting, or developing evidence-based guidelines, this article will serve as a helpful guide in navigating the hierarchy of evidence and applying it to improve patient outcomes.
Types of Reviews in Healthcare
The healthcare field encompasses various types of research reviews, each serving distinct purposes in advancing clinical practice and patient care. Understanding these different review types is essential for properly implementing evidence-based practice in nursing and healthcare settings.
Systematic Reviews
Systematic reviews represent the highest level in the evidence hierarchy, combining methodological rigor with comprehensive analysis. These reviews follow a strictly defined protocol that includes:
- Comprehensive literature searches across multiple databases
- Clear inclusion and exclusion criteria for study selection
- Standardized quality assessment of included studies
- Transparent data extraction and synthesis methods
Healthcare professionals particularly value systematic reviews because they minimize bias through their rigorous methodology and provide a comprehensive overview of available evidence on specific clinical questions.
Meta-Analyses
Meta-analyses take systematic reviews one step further by statistically combining results from multiple studies. This approach offers several advantages:
- Increased statistical power through larger combined sample sizes
- Ability to detect effects that might be missed in smaller individual studies
- Quantitative synthesis of research findings
- Enhanced precision in estimating treatment effects
Integrative Reviews
Integrative reviews combine data from both experimental and non-experimental research, providing a broader perspective on clinical issues. These reviews:
- Synthesize findings from diverse methodologies
- Address both theoretical and practical aspects
- Help identify gaps in current knowledge
- Guide future research directions
Selected Tools for Critical Appraisal and Grading the Strength of Evidence
Critical Appraisal Checklists and Worksheets
Healthcare professionals must systematically evaluate research quality using standardized tools. The Centre for Evidence-Based Medicine and various professional organizations provide comprehensive frameworks for:
Quality Assessment Tools
- CASP (Critical Appraisal Skills Programme) checklists
The CASP checklists, widely adopted in healthcare settings, provide structured approaches for evaluating different study designs. For systematic reviews, the CASP checklist examines the precision of research questions, comprehensiveness of literature searches, quality assessment of included studies, and appropriateness of methods for combining results.
- GRADE system for evidence evaluation
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system offers a comprehensive framework for evaluating evidence quality and strength of recommendations. This system considers multiple factors beyond study design, including risk of bias, inconsistency across studies, indirectness of evidence, imprecision of effect estimates, and publication bias. GRADE’s structured approach helps healthcare professionals move from evidence assessment to developing clinical recommendations, considering both the quality of evidence and the balance of benefits and harms.
- JBI critical appraisal tools
JBI critical appraisal tools, developed by the Joanna Briggs Institute, provide specialized instruments for evaluating different types of evidence. These tools include detailed criteria for assessing qualitative research, economic evaluations, diagnostic accuracy studies, and prevalence studies. The JBI approach emphasizes the importance of context in evidence evaluation, particularly relevant for nursing practice where interventions often occur within complex healthcare environments.
Implementation Considerations
- Applicability to specific clinical settings
- Resource requirements and availability
- Cultural and contextual factors
- Patient preferences and values
Grading the Strength of Evidence
Evidence grading involves systematic evaluation of multiple factors:
Quality Indicators
- Methodological rigor
- Sample size and power
- Control of confounding variables
- Consistency of findings
- Precision of results
Clinical Application Factors
- Relevance to practice
- Feasibility of implementation
- Cost-effectiveness
- Potential benefits and risks
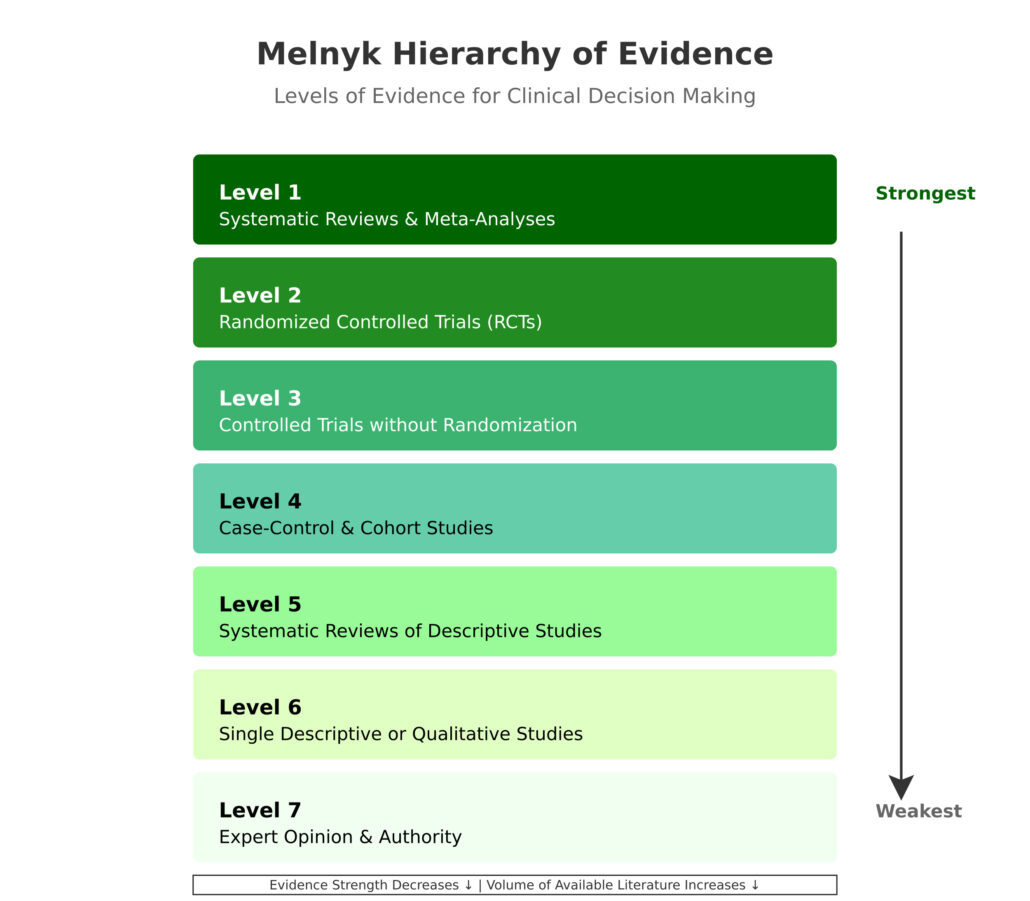
Melnyk Levels of Evidence
Evidence Pyramid (Levels of Evidence)
The Melnyk evidence pyramid serves as a cornerstone framework in evidence-based practice, providing healthcare professionals with a structured approach to evaluating research quality. This hierarchical model systematically organizes different types of evidence based on their methodological rigor, reliability, and potential for bias.
Level 1 Evidence
Level 1 evidence, comprising systematic reviews and meta-analyses, represents the pinnacle of research evidence. These comprehensive studies systematically identify, evaluate, and synthesize findings from multiple research investigations. When conducting a systematic review, researchers begin by developing a precise research question and establishing strict inclusion and exclusion criteria.
They then perform exhaustive literature searches across multiple databases, including PubMed, CINAHL, and the Cochrane Library. The methodology involves careful screening of potentially relevant studies, detailed data extraction, and quality assessment of included research. Meta-analyses take this process further by applying statistical methods to combine quantitative results across studies, providing pooled effect estimates with enhanced precision and statistical power.
The strength of Level 1 evidence lies in its comprehensive approach to minimizing bias. Systematic reviews follow the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, ensuring transparency and reproducibility. They typically include a risk of bias assessment for each included study, using tools such as the Cochrane Risk of Bias tool for randomized trials. This rigorous methodology helps healthcare professionals make informed decisions based on the totality of available evidence rather than individual studies.
Level 2 Evidence
Level 2 evidence consists of individual randomized controlled trials (RCTs), which represent the gold standard for evaluating intervention effectiveness. RCTs employ random allocation of participants to treatment groups, eliminating selection bias and ensuring that any differences between groups occur by chance alone.
The randomization process typically uses computer-generated sequences or random number tables, with allocation concealment preventing investigators from predicting group assignments. Blinding procedures, when feasible, help minimize performance and detection bias by keeping participants, healthcare providers, and/or outcome assessors unaware of treatment assignments.
The design of RCTs includes careful consideration of sample size through power calculations, clear specification of primary and secondary outcomes, and predetermined analysis plans. These studies often employ intention-to-treat analysis to maintain the benefits of randomization and provide conservative effect estimates. The CONSORT (Consolidated Standards of Reporting Trials) statement guides the reporting of RCTs, ensuring comprehensive documentation of methodology and results.
Level 3 Evidence
Level 3 evidence encompasses controlled trials without randomization, also known as quasi-experimental studies. These designs become particularly valuable when randomization proves impractical or unethical.
For example, in studying hospital-wide infection control protocols, randomly assigning different units to different protocols might be unfeasible. Instead, researchers might implement interventions sequentially across units or compare similar units that naturally adopt different approaches.
Quasi-experimental studies employ various techniques to strengthen causal inference despite the lack of randomization. These may include matching procedures to create comparable groups, difference-in-differences analysis to account for secular trends, or regression discontinuity designs that exploit natural cutoff points in treatment assignment. While these approaches cannot eliminate selection bias, they provide valuable evidence when RCTs are not possible.
Level 4 Evidence
Level 4 evidence includes case-control and cohort studies, which form the backbone of observational research. Cohort studies follow groups of individuals over time, comparing outcomes between exposed and unexposed groups.
These studies excel at investigating multiple outcomes from a single exposure and directly measuring incidence rates. Researchers must carefully consider potential confounding factors and employ appropriate statistical techniques, such as propensity score matching or multivariable regression, to adjust for baseline differences between groups.
Case-control studies, particularly useful for rare outcomes or diseases, compare individuals with a specific condition to those without it, looking backward to identify potential risk factors. These studies require careful selection of appropriate control groups and detailed assessment of exposure history to minimize recall bias. While they cannot definitively prove causation, they provide valuable insights into disease etiology and risk factors.
Level 5 Evidence
Level 5 evidence consists of systematic reviews of qualitative studies, which play a crucial role in understanding patient experiences and healthcare delivery processes. These reviews synthesize findings from multiple qualitative investigations using rigorous methodological approaches such as meta-ethnography, meta-synthesis, or meta-aggregation.
The synthesis process involves identifying key concepts across studies, translating findings between studies, and developing new interpretations that go beyond the original research while maintaining the integrity of individual study findings.
Meta-synthesis researchers employ specific methodological frameworks, such as the JBI approach or ENTREQ guidelines, to ensure transparency and rigor in their review process. They carefully evaluate the methodological quality of included studies using tools like the CASP qualitative checklist or the JBI Qualitative Assessment and Review Instrument. The synthesis process often reveals patterns and themes that might not be apparent in individual studies, providing deeper insights into phenomena relevant to healthcare practice.
Level 6 Evidence
Level 6 encompasses single qualitative or descriptive studies, which provide rich, detailed explorations of healthcare phenomena. These studies employ various methodological approaches, including phenomenology, grounded theory, ethnography, and narrative inquiry.
Phenomenological studies explore the lived experiences of patients or healthcare providers, using in-depth interviews and careful analysis to uncover the essence of specific healthcare experiences. Grounded theory studies systematically develop theoretical explanations of social processes in healthcare settings, using constant comparative analysis and theoretical sampling to build robust conceptual frameworks.
Ethnographic research in healthcare settings involves prolonged engagement with specific clinical environments or cultural groups, combining observation, interviews, and document analysis to understand healthcare delivery within its cultural context. Narrative inquiry focuses on collecting and analyzing stories from patients, families, or healthcare providers, offering insights into how people make sense of their healthcare experiences and interactions with the healthcare system.
Level 7 Evidence
Level 7 evidence, comprising expert opinion and expert committee reports, while positioned at the base of the hierarchy, serves an important function in healthcare decision-making. Expert opinions often draw from years of clinical experience and a deep understanding of specific practice areas. These insights become particularly valuable when higher-level evidence is lacking or when applying research findings to complex clinical situations. Expert committees typically follow structured processes for developing consensus, such as the Delphi technique or nominal group process, to generate practice guidelines and recommendations.
Hierarchy of Evidence for Qualitative Studies
The qualitative evidence hierarchy recognizes the unique contributions of different qualitative methodologies to healthcare knowledge. Meta-syntheses of qualitative studies occupy the highest level, providing comprehensive integration of findings across multiple studies. These syntheses employ sophisticated analytical approaches to maintain the richness of qualitative findings while identifying overarching patterns and themes.
Individual qualitative studies vary in their methodological approaches and contributions to evidence. Phenomenological studies offer deep insights into lived experiences, while grounded theory studies develop theoretical frameworks to explain social processes in healthcare settings. Ethnographic research provides a detailed understanding of cultural contexts affecting healthcare delivery, and narrative studies illuminate personal meanings and experiences of health and illness.
Hierarchy of Evidence for Quantitative Questions
The quantitative evidence hierarchy emphasizes methodological rigor and control of potential biases. Systematic reviews and meta-analyses of randomized controlled trials provide the most reliable evidence for intervention effectiveness. These reviews employ sophisticated statistical methods to combine results across studies, including random-effects models to account for between-study heterogeneity and meta-regression to explore sources of variation in treatment effects.
Individual experimental studies follow strict methodological guidelines to ensure internal validity. Randomized controlled trials employ various design features to minimize bias, such as allocation concealment, blinding procedures, and intention-to-treat analysis. Cluster randomized trials address situations where individual randomization is impractical, such as hospital-wide interventions, while accounting for the clustering effect in their analysis.
Best Types of Evidence for Different Clinical Questions
Different clinical questions require different types of evidence to provide optimal answers. Therapy questions are best addressed by randomized controlled trials and their systematic reviews, as these designs can establish causal relationships between interventions and outcomes. Diagnostic questions require studies comparing new tests against established reference standards, ideally in prospective, blinded designs.
Prognostic questions are best answered by cohort studies that follow patients over time, while etiology questions often rely on case-control studies, particularly for rare outcomes. Questions about patient experiences and preferences typically require qualitative research approaches. Understanding these relationships helps healthcare professionals select and apply the most appropriate evidence for specific clinical questions.
Research Designs and Methods in Evidence-Based Practice
Understanding research design complexities helps healthcare professionals evaluate evidence quality and applicability. Experimental designs, particularly randomized controlled trials, employ sophisticated methods to control potential biases. Beyond simple randomization, modern RCTs often utilize adaptive designs that allow for predetermined modifications based on interim analyses while maintaining statistical rigor.
Advanced randomization techniques like stratified randomization and minimization help ensure balance across important prognostic factors. Block randomization maintains treatment balance in smaller studies or when sequential enrollment occurs. Cluster randomization addresses situations where individual randomization isn’t feasible, requiring specialized statistical approaches to account for intracluster correlation.
Quasi-experimental designs employ creative approaches to strengthen causal inference when randomization isn’t possible. Interrupted time series analyses use multiple observations before and after intervention implementation to control for secular trends. Regression discontinuity designs exploit natural cutoff points in treatment assignment to approximate randomized experiments. Difference-in-differences analyses help control for unmeasured confounding that remains constant over time.
Conclusion
The Melnyk levels of evidence provide an essential framework for evidence-based practice in nursing and healthcare. By understanding and applying these levels of evidence, healthcare professionals can make better-informed decisions and provide optimal patient care. The system helps researchers and practitioners evaluate, rank, and adapt evidence to their clinical needs.
Our academic writing services offer comprehensive support for nursing professionals seeking help with Melnyk levels of evidence assignments. Whether you need assistance with research design, evidence evaluation, or implementing evidence-based practice in your clinical setting, our experts can provide the guidance you need.
FAQs
How does the Melnyk framework differ from other evidence hierarchies used in healthcare?
The Melnyk levels of evidence specifically incorporate both quantitative and qualitative research methodologies, providing a comprehensive framework that recognizes the value of different types of evidence in healthcare decision-making. This approach distinguishes it from earlier hierarchies that focused primarily on quantitative research.
What role do systematic reviews play in the Melnyk levels of evidence, and why are they considered the highest level?
Systematic reviews occupy the highest level because they provide a comprehensive synthesis of multiple research studies, employing rigorous methodological approaches to minimize bias and generate reliable conclusions. Their systematic approach to identifying, evaluating, and combining research findings provides the most trustworthy evidence for clinical decision-making.
How can healthcare professionals effectively integrate lower levels of evidence when higher-level evidence is unavailable?
When higher-level evidence is unavailable, healthcare professionals should carefully evaluate lower-level evidence using standardized critical appraisal tools, consider the context of their specific clinical situation, and combine this with their professional expertise and patient preferences to make informed decisions.
What considerations should guide the application of evidence-based practice in specialty nursing areas?
Specialty nursing areas require careful consideration of patient populations, clinical contexts, and specific practice requirements. Healthcare professionals should evaluate evidence applicability to their specialty, consider resource availability, and assess implementation feasibility within their specific practice environment.
How do qualitative studies contribute to evidence-based practice within the Melnyk framework?
Qualitative studies provide essential insights into patient experiences, healthcare delivery processes, and implementation challenges. While they occupy lower levels in the evidence hierarchy, their contributions to understanding complex healthcare phenomena and patient perspectives are crucial for comprehensive evidence-based practice.
What is the Melnyk hierarchy?
The Melnyk hierarchy is a ranking system for medical research evidence, ranging from Level 1 (strongest) to Level 7 (weakest). Healthcare professionals use it to evaluate research reliability when making clinical decisions.
Are there five levels of evidence?
No, there are seven levels, not five. The confusion often comes from older evidence hierarchies that use five levels. The Melnyk hierarchy uses seven distinct levels to provide a more detailed classification of evidence types.
What’s the difference between Levels 1, 2, and 3?
- Level 1: Systematic reviews and meta-analyses that combine multiple research studies
- Level 2: Individual randomized controlled trials (RCTs) with randomized participants
- Level 3: Controlled trials without randomization, similar to RCTs but lacking random assignment
What counts as Level 7 evidence?
Level 7 evidence includes:
- Expert opinions
- Committee reports
- Consensus statements
- Clinical experience
It’s the lowest level because it relies on expertise rather than systematic research.
Can you rank all seven levels from strongest to weakest?
Here’s the complete hierarchy:
- Systematic reviews & meta-analyses
- Randomized controlled trials
- Controlled trials without randomization
- Case-control & cohort studies
- Systematic reviews of descriptive studies
- Single descriptive or qualitative studies
- Expert opinion & authority

 Add Instructions
Add Instructions
