Hypotonic vs Hypertonic Solution: Clear Difference Between Hypotonic, Isotonic, and Hypertonic IV Fluids
Fluid balance is a fundamental aspect of human physiology, directly influencing cellular function, tissue perfusion, and overall homeostasis. In clinical practice, understanding the movement of water across the cell membrane via osmosis is essential for safe and effective intravenous therapy. Hypotonic, hypertonic, and isotonic solutions are types of IV fluids frequently used in nursing care to restore or maintain fluid balance, yet their effects on cells and body fluids differ significantly depending on solute concentration.
A hypertonic solution contains a higher concentration of solutes than the fluid inside the cell, which causes water to move out of cells, leading to shrinkage or crenation. Conversely, a hypotonic solution has a lower concentration of solutes compared to the intracellular environment, resulting in water moving into cells, causing them to swell and, in extreme cases, rupture. Isotonic solutions, however, have a solute concentration approximately equal to that inside cells and the extracellular fluid, producing minimal net movement of water and maintaining equilibrium between inside and outside the cell.
For nurses, accurately identifying the tonicity of a solution and understanding how it affects red blood cells, plasma, and overall body fluids is critical. The choice of IV fluid—whether hypertonic, hypotonic, or isotonic—can influence patient outcomes, from correcting dehydration or hyponatremia to preventing fluid overload. Furthermore, these principles of osmosis and solute concentration extend beyond cellular responses; they also guide clinical decisions regarding sodium management, fluid resuscitation, and monitoring for complications in various care settings.
This article provides a comprehensive overview of hypotonic vs hypertonic solutions, detailing their differences, physiological effects, and practical nursing considerations. By exploring how water moves inside and outside the cell in response to different solute concentrations, examining common IV fluids used in clinical practice, and highlighting real-world scenarios, nurses can develop a clearer understanding of how to safely implement fluid therapy. Ultimately, mastery of these concepts equips nursing students and practitioners with the knowledge to make informed decisions about IV fluid administration, ensuring both cellular integrity and patient safety.
Understanding Hypertonic, Hypotonic, and Isotonic Solutions
Intravenous (IV) fluid therapy is a critical component of patient care, and the choice between hypertonic, hypotonic, and isotonic solutions depends on the patient’s clinical condition, electrolyte balance, and the desired movement of water across the cell membrane. The behavior of water in response to solute concentration differences between the inside and outside of the cell is governed by osmosis, a fundamental physiological principle. Understanding these solutions’ effects on red blood cells, plasma, and body fluids is essential for safe and effective fluid management.
What is a Hypertonic Solution and How Does it Affect Cells?
A hypertonic solution is a type of solution that contains a higher concentration of solutes than the intracellular fluid. This means that the concentration of solute outside the cell is greater than inside the cell, creating an osmotic gradient. When a cell is placed in a hypertonic solution, water moves out of the cell via osmosis, from the area of lower solute concentration (inside the cell) to higher solute concentration (outside the cell). This net movement of water causes the cell to shrink, a process known as crenation in red blood cells or plasmolysis in plant cells.
Clinical relevance: Hypertonic IV solutions are used to draw water out of cells into the extracellular space, which can be beneficial in specific conditions, such as reducing cerebral edema, correcting hyponatremia, or treating hypovolemia when isotonic fluids are insufficient. However, improper administration of hypertonic IV solutions can lead to cellular dehydration, vascular irritation, and osmotic demyelination syndrome in severe cases.
Examples of hypertonic solutions in IV therapy include:
- 3% NaCl (hypertonic saline) – often used in critical care settings to treat severe hyponatremia.
- 5% NaCl – rarely used, typically under strict monitoring.
- Dextrose 10% in water (D10W) – initially isotonic in the bag but acts as hypertonic after metabolism of dextrose.
- 5% dextrose in 0.9% NaCl (D5NS) – used when a hypertonic solution is needed for fluid and caloric supplementation.
In nursing practice, hypertonic IV fluids require careful monitoring of blood pressure, sodium levels, and neurological status due to the significant osmotic shifts they induce.
What is a Hypotonic Solution and How Does it Affect Cells?
A hypotonic solution has a lower concentration of solutes compared to the intracellular fluid. This means that the solute concentration outside the cell is less than inside, creating a gradient that favors the movement of water into the cell via osmosis. As water moves inside, cells swell and may eventually undergo lysis if the osmotic gradient is extreme.
Clinical relevance: Hypotonic IV solutions are primarily used to rehydrate cells in cases of intracellular dehydration, such as in hypernatremia or diabetic ketoacidosis, where the cells have lost water. However, care must be taken because excessive administration can lead to cellular swelling, edema, or hyponatremia, particularly in the brain.
Examples of hypotonic IV solutions include:
- 0.45% NaCl (half-normal saline) – often used for patients requiring maintenance fluids.
- 0.33% NaCl – rarely used, typically in pediatrics.
- 0.225% NaCl – used in very specific clinical scenarios under monitoring.
In nursing practice, hypotonic solutions should not be used for patients at risk of increased intracranial pressure or those with burns and trauma because the water influx can exacerbate tissue edema.
Isotonic Solutions: Maintaining Fluid Balance
An isotonic solution is a solution in which the concentration of solutes is approximately equal to that inside the cell and in extracellular fluid, producing minimal osmotic movement. In other words, water movement across the cell membrane is balanced, and cells maintain their normal shape and volume.
Clinical relevance: Isotonic IV solutions are commonly used for fluid resuscitation, restoring extracellular volume, and maintaining adequate tissue perfusion. They are generally safe for most patients, including those in hypovolemic shock, because they do not cause cells to shrink or swell.
Examples of isotonic IV solutions include:
- 0.9% NaCl (normal saline) – widely used for fluid replacement and IV medication administration.
- Lactated Ringer’s solution – contains sodium, potassium, calcium, and lactate, making it suitable for surgical, trauma, and burn patients.
- 5% dextrose in water (D5W) – initially isotonic but metabolizes to hypotonic in the body.
Compared to hypertonic IV fluids, isotonic solutions do not shift water from cells into the extracellular space, making them preferable for general hydration. Compared to hypotonic IV fluids, they avoid cellular swelling, making them safe for patients at risk of edema. For nursing students and practitioners, recognizing when to use isotonic IV solutions versus hypertonic or hypotonic solutions is critical to patient safety.
Osmosis and Cellular Response to IV Fluids
Understanding osmosis is central to nursing practice, particularly when administering IV fluids. Osmosis describes the movement of water across the cell membrane from areas of lower concentration of solute to areas of higher concentration of solute. The response of cells to hypertonic, hypotonic, and isotonic solutions directly influences red blood cells, plasma, tissue fluid, and overall body fluids, guiding safe fluid therapy decisions.
How Hypertonic, Hypotonic, and Isotonic Solutions Affect Cells
A hypertonic solution contains a higher concentration of solutes than inside the cell. When a cell is placed in a hypertonic environment, water moves out of the cell via osmosis, causing the cell to shrink, a process known as crenation in red blood cells. This movement also increases the extracellular fluid volume, which can be useful clinically, such as in the treatment of cerebral edema or severe hyponatremia. Common hypertonic IV solutions include 3% NaCl, 5% NaCl, D5NS, and D10W. Nursing students must recognize that hypertonic solutions cause water to rush out of the cell, making careful monitoring essential to prevent complications like dehydration hypertonic and hypotonic, vascular irritation, or neurological issues.
In contrast, a hypotonic solution has a lower concentration of solutes than the fluid inside the cell. When cells are exposed to a hypotonic solution, water moves into the cell via osmosis, which causes cells to swell and may lead to lysis if extreme. This property makes hypotonic IV solutions, such as 0.45% NaCl or 0.33% NaCl, effective for rehydrating cells, particularly in hypernatremia or intracellular dehydration. However, administering hypotonic solutions in patients with increased intracranial pressure, burns, or trauma may exacerbate tissue edema or cellular swelling, highlighting the importance of careful clinical assessment.
Isotonic solutions, including 0.9% NaCl and Lactated Ringer’s, have a solute concentration similar to that inside the cell. The net movement of water across the cell membrane is minimal, maintaining equilibrium between inside and outside the cell. Cells retain their shape, red blood cells remain stable, and tissue fluid is preserved. Isotonic IV solutions are ideal for fluid resuscitation, maintaining blood pressure, and general hydration, making them one of the safest and most commonly used IV fluids in clinical practice. In some cases, D5W may initially act as isotonic but becomes hypotonic after metabolism of dextrose in the body, demonstrating the dynamic nature of IV fluid behavior.
Water Movement in Response to Solute Concentration
The movement of water is dictated by the concentration gradient between the intracellular and extracellular environment. Water naturally flows from an area with lower concentration of solute to an area with higher concentration of solute to equalize solute levels. For example:
- A cell in a hypertonic solution loses water, causing shrinkage.
- A cell in a hypotonic solution gains water, resulting in swelling.
- Cells in isotonic solutions remain stable, as the solute concentration is balanced inside and outside the cell.
This principle underpins the selection of IV fluids, allowing nurses to anticipate the effect on red blood cells, tissue fluid, and plasma volume, and prevent complications associated with improper fluid administration.
Effects on Red Blood Cells, Plasma, and Tissue Fluid
Hypertonic, hypotonic, and isotonic solutions influence blood components and body fluids differently. Hypertonic fluids increase extracellular volume while causing cellular dehydration, hypotonic fluids increase intracellular volume and can produce tissue edema, and isotonic fluids maintain equilibrium, avoiding significant shifts. These effects are particularly critical in patients with cardiovascular compromise, renal impairment, or electrolyte imbalances, where precise fluid management is essential.
Differences in Plant vs Animal Cells
While plant cells are not typically a clinical focus, understanding their response highlights the protective role of a cell wall. In hypertonic solutions, plant cells undergo plasmolysis, where the cell membrane pulls away from the cell wall, while in hypotonic solutions, the cells become turgid but rarely lyse due to structural support. Animal cells, such as red blood cells, lack this support and are more susceptible to shrinkage in hypertonic solutions or swelling in hypotonic solutions, which is directly relevant to patient care.
The Role of Osmosis in Fluid Therapy
Osmosis guides IV fluid choice by predicting how water will move between compartments. Practical considerations include:
- Dehydration hypertonic and hypotonic: Hypertonic dehydration requires isotonic or hypotonic fluids to restore water to cells, whereas hypotonic dehydration requires careful use of hypertonic fluids to correct solute imbalances.
- Fluid overload: Excess isotonic or hypotonic solutions may cause edema, affecting tissue perfusion and organ function.
- Clinical monitoring: Nurses assess muscle function, blood pressure, sodium levels, and fluid balance to ensure safe IV fluid administration.
- Nursing school practice: Students learn to predict net movement of water in response to various solutions, enhancing their ability to manage real-world clinical scenarios.
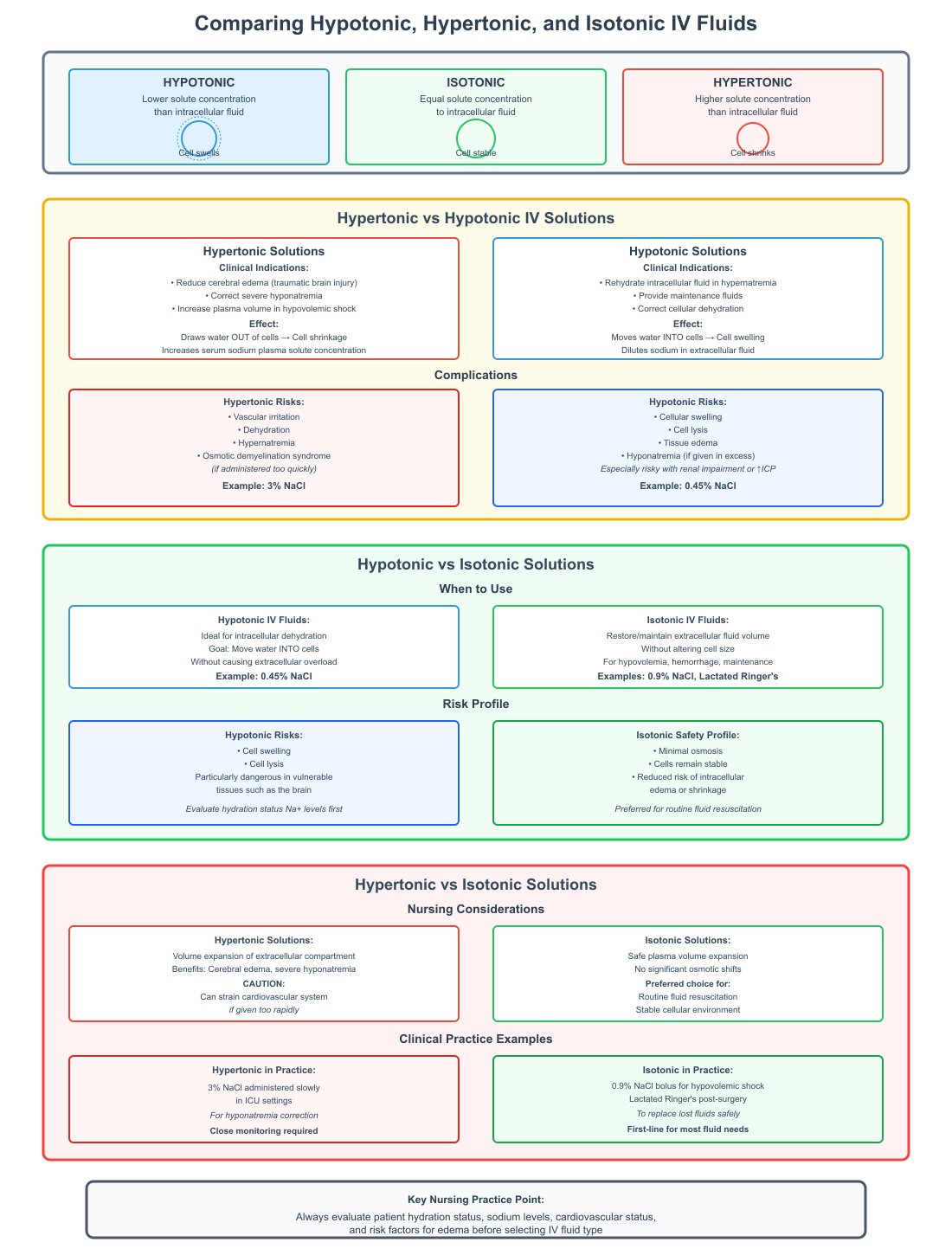
Comparing Hypotonic, Hypertonic, and Isotonic IV Fluids
Effective fluid therapy requires not only understanding osmosis and cellular response but also knowing how different IV fluids compare in their clinical use, solute effects, and risks. Nurses must differentiate between hypertonic vs hypotonic, hypotonic vs isotonic, and hypertonic vs isotonic IV fluids to provide safe and effective care.
Hypertonic vs Hypotonic IV Solutions
Clinical differences and indications:
Hypertonic and hypotonic IV fluids have opposite effects on cells and body fluids. A hypertonic solution has a higher concentration of solutes than the intracellular fluid, which draws water out of cells into the extracellular space, causing cell shrinkage. In contrast, a hypotonic solution has a lower concentration of solutes than inside the cell, moving water into the cells, causing them to swell.
- Hypertonic IV solutions are indicated in situations where it is necessary to:
- Reduce cerebral edema after traumatic brain injury.
- Correct severe hyponatremia where sodium is critically low.
- Increase plasma volume in hypovolemic shock when isotonic fluids alone are insufficient.
- Hypotonic IV solutions are used to:
- Rehydrate intracellular fluid in hypernatremia.
- Provide maintenance fluids when daily fluid and electrolyte requirements need to be met.
- Correct cellular dehydration without causing excessive extracellular expansion.
Sodium and other solute effects in the body:
- Hypertonic solutions increase serum sodium and raise solute concentration in plasma, which can help draw water from intracellular to extracellular compartments.
- Hypotonic solutions dilute sodium levels in extracellular fluid, which can risk hyponatremia if administered in excess.
- Both types of solutions influence potassium, glucose, and other solutes, making careful monitoring critical in clinical care.
Complications:
- Hypertonic IV fluids can cause vascular irritation, dehydration, hypernatremia, and osmotic demyelination syndrome if given too quickly.
- Hypotonic IV fluids carry risks of cellular swelling, lysis, and tissue edema, especially in patients with renal impairment or increased intracranial pressure.
Hypotonic vs Isotonic Solutions
When to use hypotonic vs isotonic IV fluids:
- Hypotonic solutions are ideal for patients with intracellular dehydration, where the goal is to move water into the cells without causing extracellular overload.
- Isotonic solutions are used when the goal is to restore or maintain extracellular fluid volume without altering cell size, such as in hypovolemia, hemorrhage, or routine IV maintenance.
Risks:
- Hypotonic IV fluids may lead to cell swelling and lysis, particularly in vulnerable tissues such as the brain.
- Isotonic IV fluids produce minimal osmosis, keeping cells stable and reducing the risk of intracellular edema or shrinkage.
Examples in clinical practice:
- Hypotonic IV fluids: 0.45% NaCl (half-normal saline).
- Isotonic IV fluids: 0.9% NaCl, Lactated Ringer’s.
- Nursing students and practitioners should evaluate patient hydration status, sodium levels, and risk factors for edema before selecting either solution.
Hypertonic vs Isotonic Solutions
Nursing considerations:
- Hypertonic IV solutions cause volume expansion of the extracellular compartment, which can benefit patients with cerebral edema or severe hyponatremia but can strain the cardiovascular system if given too rapidly.
- Isotonic IV solutions expand plasma volume safely without significant osmotic shifts, making them the preferred choice for routine fluid resuscitation.
Clinical examples:
- Hypertonic IV fluids: 3% NaCl administered slowly in ICU settings for hyponatremia correction.
- Isotonic IV fluids: 0.9% NaCl bolus for hypovolemic shock or Lactated Ringer’s post-surgery to replace lost fluids.
Nurses must monitor serum electrolytes, fluid balance, and neurological status when using hypertonic IV fluids, while isotonic IV solutions generally require standard fluid monitoring. Understanding the differences in solute concentration and osmotic effect allows nurses to anticipate cellular and systemic responses, preventing complications such as edema, dehydration, or electrolyte imbalances.
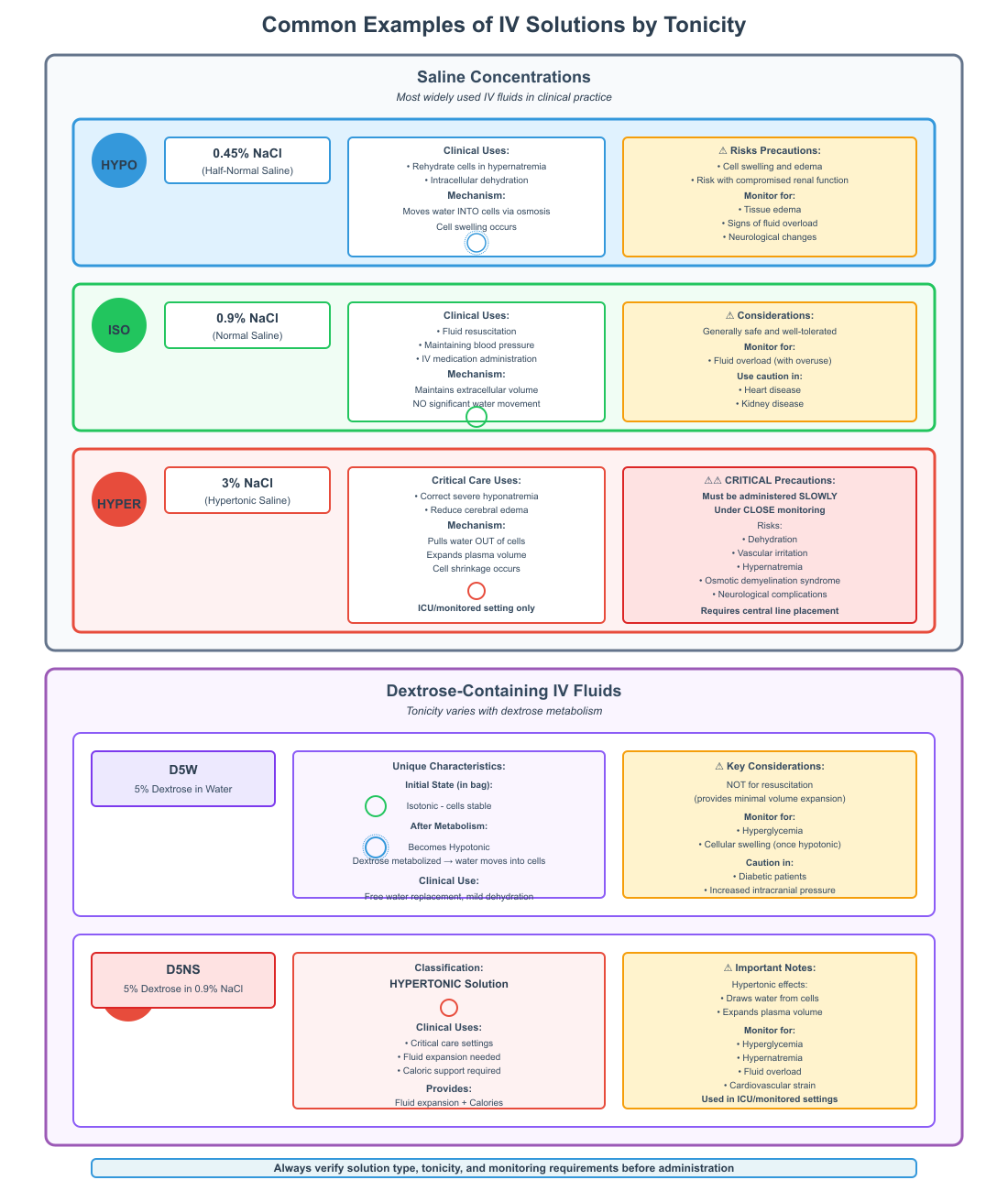
Practical Applications and Clinical Examples
The administration of hypertonic, hypotonic, and isotonic IV fluids is a cornerstone of patient care, particularly in hospital and critical care settings. Nurses must understand how these solutions affect cells and body fluids via osmosis, their clinical indications, and potential complications to safely manage patient hydration and electrolyte balance.
Common Examples of Hypertonic, Hypotonic, and Isotonic Solutions
Saline concentrations are the most widely used IV fluids, and understanding their tonicity is essential:
- 0.45% NaCl (half-normal saline): This hypotonic solution is used to rehydrate cells when intracellular dehydration occurs, such as in hypernatremia. It moves water into cells via osmosis, but excessive administration can lead to cell swelling and edema, particularly in patients with compromised renal function.
- 0.9% NaCl (normal saline): This isotonic solution maintains extracellular volume without causing significant water movement across the cell membrane. It is commonly used for fluid resuscitation, maintaining blood pressure, or IV medication administration. It is generally safe, but overuse may contribute to fluid overload, particularly in patients with heart or kidney disease.
- 3% NaCl: This hypertonic solution is used to correct severe hyponatremia or reduce cerebral edema. Water is pulled out of cells, expanding plasma volume. Hypertonic IV fluids must be administered slowly and under close monitoring to prevent dehydration, vascular irritation, and neurological complications.
Dextrose-containing IV fluids also vary in tonicity:
- D5W (5% dextrose in water) initially behaves as an isotonic solution in the bag but becomes hypotonic once dextrose is metabolized, allowing water to move into cells.
- D5NS (5% dextrose in 0.9% NaCl) is hypertonic and provides both fluid expansion and caloric support, often used in critical care settings.
Complications and Nursing Interventions
Hypertonic IV fluid risks:
- Dehydration of cells as water moves out, potentially worsening intracellular deficits.
- Vascular irritation, particularly with rapid administration of concentrated solutions.
- Hypernatremia and osmotic demyelination if serum sodium rises too quickly.
Nursing interventions:
- Monitor serum electrolytes, vital signs, and neurological status.
- Administer hypertonic IV fluids slowly, using a central line if needed.
- Educate patients on symptoms of dehydration such as dizziness or hypotension.
Hypotonic IV fluid risks:
- Cellular swelling, which can increase intracranial pressure.
- Edema, particularly in lungs or peripheral tissues.
- Hyponatremia due to dilution of extracellular sodium.
Nursing interventions:
- Assess fluid status and weight daily.
- Monitor for signs of edema and neurological changes.
- Avoid hypotonic IV fluids in patients with burns, trauma, or head injuries.
Isotonic IV fluid safety and monitoring:
- Generally well tolerated with minimal cellular effect.
- Monitor for fluid overload, especially in patients with heart failure or renal impairment.
- Adjust infusion rates according to urine output and blood pressure.
Real-World Scenarios and Case Examples
- Dehydration scenario:
- A patient with hypernatremia due to excessive vomiting may receive 0.45% NaCl to rehydrate cells, restoring intracellular fluid balance. Nursing monitoring focuses on electrolytes and signs of edema.
- Fluid overload scenario:
- A post-operative patient with oliguria may develop pulmonary edema if infused with excessive 0.9% NaCl. Nursing intervention includes slowing the infusion and monitoring lung sounds, oxygen saturation, and daily weights.
- Critical care scenario:
- A patient with cerebral edema may require 3% NaCl. Nurses must administer via central line, monitor serum sodium levels, and watch for neurological changes, reflecting the hypertonic movement of water out of cells.
- Dextrose-containing solution scenario:
- A malnourished patient receiving D5W requires monitoring for cellular hydration, blood glucose, and serum electrolytes, as the solution transitions from isotonic to hypotonic once glucose is metabolized.
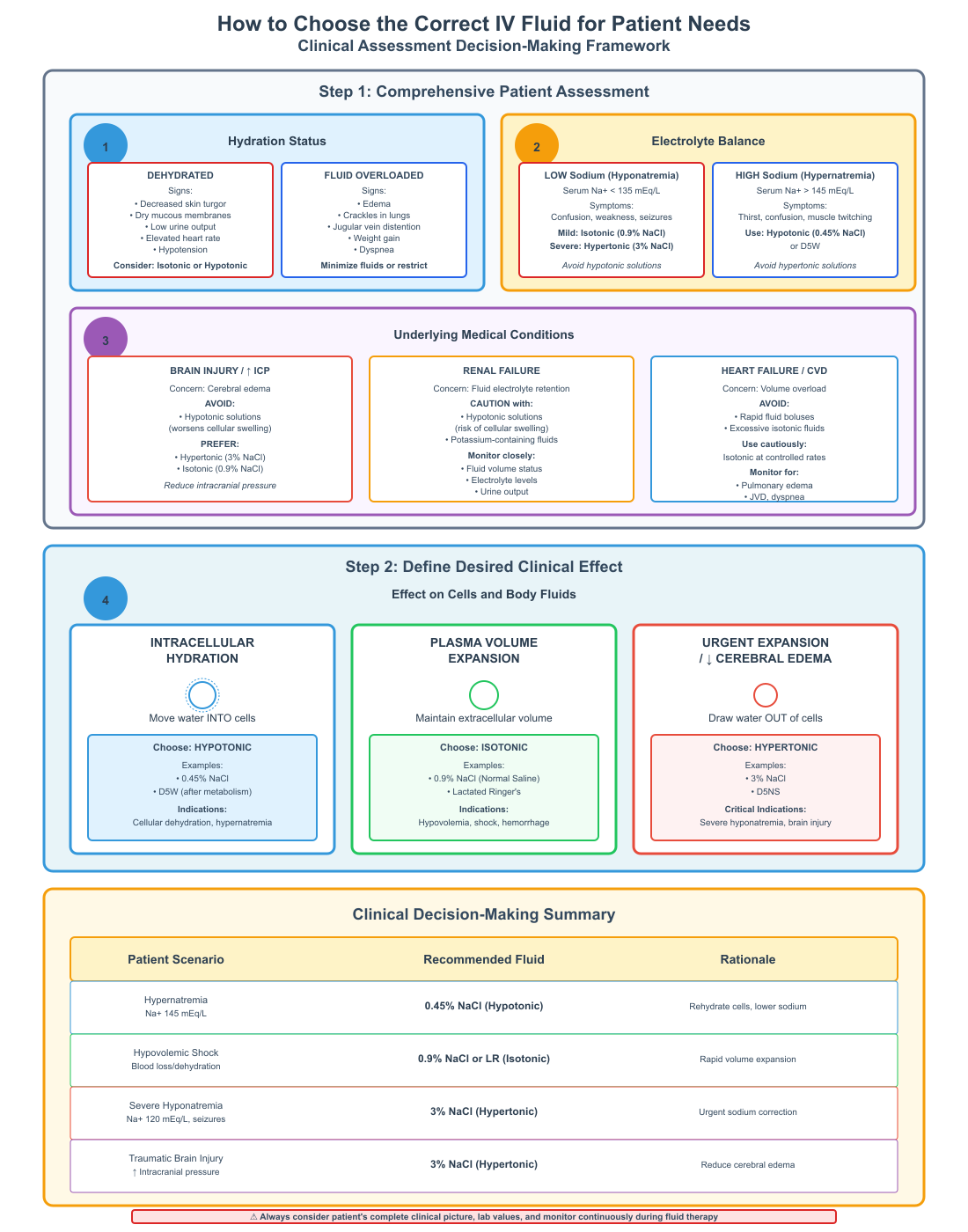
How to Choose the Correct IV Fluid for Patient Needs
Choosing the appropriate IV fluid involves assessing:
- Patient’s hydration status (dehydrated vs fluid overloaded).
- Electrolyte balance, particularly sodium and potassium.
- Underlying conditions, such as brain injury, renal failure, or cardiovascular compromise.
- Desired effect on cells and body fluids (hydration vs plasma expansion).
Reverse osmosis and water movement are crucial considerations: nurses anticipate how water will shift inside and outside the cell to prevent cellular damage or edema. For example, in hypertonic dehydration, water moves from cells into plasma, which can restore blood pressure but shrink cells, requiring careful monitoring.
Conclusion
Understanding the differences between hypotonic, hypertonic, and isotonic IV fluids is fundamental to safe and effective nursing practice. These solutions influence cellular hydration, plasma volume, and overall body fluid balance through the principle of osmosis, where water moves across the cell membrane in response to differences in solute concentration. The ability to predict how a cell in a hypertonic or hypotonic solution will respond enables nurses to select the most appropriate IV fluid for specific clinical scenarios.
Hypertonic solutions draw water out of cells, increasing extracellular volume and helping manage conditions like cerebral edema or severe hyponatremia, but they carry risks such as dehydration and vascular irritation. Hypotonic solutions move water into cells, restoring intracellular hydration, yet may cause edema or hyponatremia if misused. Isotonic solutions maintain equilibrium, stabilizing red blood cells, tissue fluid, and plasma without significant osmotic shifts, making them the safest choice for routine fluid replacement and volume maintenance.
For nurses, the practical application of this knowledge requires not only understanding theoretical principles but also translating them into real-world clinical decisions. Monitoring electrolytes, vital signs, urine output, and patient symptoms, and considering patient-specific factors like renal function, cardiovascular status, and neurological condition, are essential for preventing complications and optimizing outcomes.
By mastering the distinctions between hypotonic, hypertonic, and isotonic IV fluids, nursing students and practitioners gain a critical skill set that enhances patient safety, supports precise fluid therapy, and fosters a deeper comprehension of how fluids interact with cells and body systems. Ultimately, this knowledge empowers nurses to make evidence-based decisions that maintain cellular integrity, correct fluid imbalances, and improve patient care across a wide range of clinical settings.
Frequently Asked Questions
What is the difference between hypertonic and hypotonic?
- A hypertonic solution has a higher concentration of solutes than the fluid inside the cell, causing water to move out of the cell, leading to cell shrinkage (crenation).
- A hypotonic solution has a lower concentration of solutes than the fluid inside the cell, causing water to move into the cell, leading to cell swelling or possible lysis.
Does hypotonic mean shrink or swell?
- Hypotonic means swell. Cells placed in a hypotonic solution gain water via osmosis, increasing their size.
How to remember hypotonic vs hypertonic?
- “Hyper → H for high”: Hypertonic has high solute, water leaves the cell → shrink.
- “Hypo → L for low”: Hypotonic has low solute, water enters the cell → swell.
- A quick mnemonic: “Hyper makes cells smaller, Hypo makes cells happy (big)”.
What is the difference between hypertonic and hypotonic blood cells?
- In hypertonic blood, red blood cells shrink (crenate) due to water leaving the cell.
- In hypotonic blood, red blood cells swell and may burst (hemolyze) due to water entering the cell.
